In a world where TikTok obstacles and infant style patterns guideline, why not include "Baby Covid Vaccines" to the mix? Yes, you check out that right, since what could be trendier than children sporting the most current vaccine on the play ground? Hey, do not fret; we're sure their small arms can manage it.
The United States drug regulators have actually just recently provided the green light for brand-new Covid vaccinations to be administered to people as young as 6 months old. Dr. Ladapo pointed to research studies that have actually revealed a decrease in the efficiency of Covid vaccines over time. He highlighted research study that recommended a possible link in between these vaccines and heart issues, such as heart swelling.
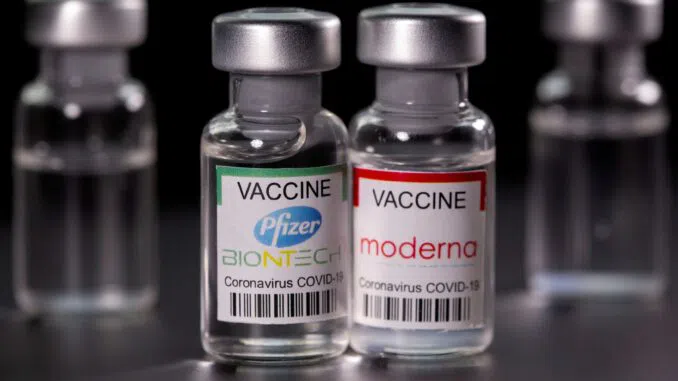
The U.S. Food and Drug Administration (FDA) has actually okayed for Covid shots from Moderna and Pfizer to be administered to people as young as 6 months old, beginning later on this month. This permission comes in the middle of a background of issues concerning the negative effects of Covid shots and their efficiency in avoiding infection.
Dr. Peter Marks, a leading FDA authorities, worried the significance of vaccination for public health, especially in protecting versus the extreme repercussions of Covid-19, consisting of hospitalization and death. The FDA had actually formerly authorized these vaccines for those aged 12 and older, with emergency situation permission given for the 6-month to 11-year age group.
These freshly licensed shots particularly target the XBB.1.5 subvariant of the Omicron infection version. It's worth keeping in mind that this subvariant has actually mainly been superseded by more recent pressures, consisting of EG.5, according to the U.S. Centers for Disease Control and Prevention (CDC).
Permission Amidst Data Gaps
One striking element of this permission is the absence of extensive information from scientific trials. Both Moderna and Pfizer have actually asserted the effectiveness of their brand-new shots versus EG.5 and other more recent versions, relying on preclinical information and immune action signs.
The CDC prepares to assemble with its consultants to figure out which populations must get these brand-new vaccines.: Because absolutely nothing states fashion-forward like a vaccine sticker label on a diaper!
In a world where TikTok obstacles and child style patterns guideline, why not include "Baby Covid Vaccines" to the mix? Yes, you check out that right, since what could be trendier than infants sporting the most current vaccine on the play area? Dr. Ladapo pointed to research studies that have actually revealed a decrease in the efficiency of Covid vaccines over time. The FDA had actually formerly authorized these vaccines for those aged 12 and older, with emergency situation permission approved for the 6-month to 11-year age group. The CDC prepares to assemble with its consultants to identify which populations must get these brand-new vaccines.
Free Speech and Alternative Media are under attack by the Deep State. We need your support to survive.
Please Contribute via GoGetFunding